Cost and emissions intensity of hydrogen from thermal pyrolysis of natural gas in BC
Cinthia Serrato1*, Andrew Rowe1, 1 Institute for Integrated Energy Systems, University of Victoria, British Columbia, Canada. * Correspondence: cserratoarias@uvic.ca
Key messages
- Developed and compared three methane pyrolysis processes for 50 tH2/day
- Levelized cost of hydrogen ranges from 2.30-4.30 USD/kg
- Carbon dioxide emissions range from 4.1-0.1 kgCO2/kgH2
Importance: Low-carbon and low-cost hydrogen is needed for deep decarbonisation
The future of energy systems presents some challenges in providing carbon-neutral and low-emission fuels. Hydrogen is a fuel that can potentially bridge some of the energy system gaps in the transportation and industrial sectors (Brandon & Kurban, 2017; Sánchez-Bastardo et al., 2021). To date, hydrogen generation techniques have been limited, with most of its production coming from steam-methane reforming (SMR); however, this technology exhibits a high carbon intensity of 9-14 kgCO2/kgH2, despite a low minimum cost of 1-2 USD/kgH2. (Incer-Valverde et al., 2023; Parkinson et al., 2018; Timmerberg et al., 2020). Other potential technologies have been explored to generate hydrogen more sustainably, such as electrolysis, which aims to produce clean hydrogen when its electricity is derived from low-carbon sources such as renewable energy, although its major drawback is its high cost around 4-8 USD/kgH2 (Incer-Valverde et al., 2023; Parkinson et al., 2018).
Besides SMR and electrolysis, other methods exist to produce hydrogen, one of which is methane pyrolysis (MP). This process consists of thermally cracking the methane molecule to produce hydrogen and solid carbon (Korányi et al., 2022; Marquardt et al., 2020). Solid carbon can be a valuable co-product and removes the challenges of carbon dioxide capture, transport, and sequestration which would be required with SMR. Energy input is needed to drive the process. The reactor itself needs high temperature heat, and power is needed for gas conditioning and flow control. MP processes that use methane or natural gas for heat input produce carbon dioxide due to combustion; mitigating these emissions is possible with carbon capture or by using electrical heating.
This study analyzes three methane pyrolysis process designs: (1) gas heating, (2) gas heating and carbon capture, (3) electric heating. The key performance metrics are total levelized cost of hydrogen and carbon intensity of hydrogen. A sensitivity analysis is performed on key parameters including possible revenues from carbon co-product.
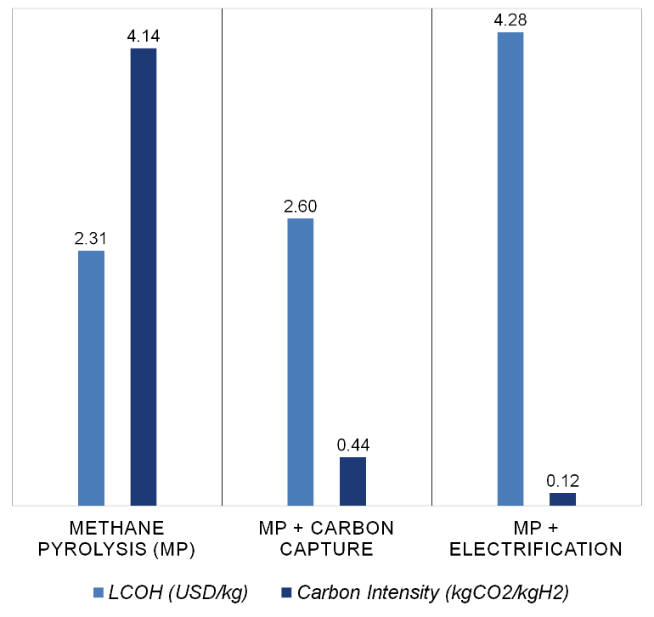
Figure 1. Hydrogen cost and emissions intensity for three methane pyrolysis processes.
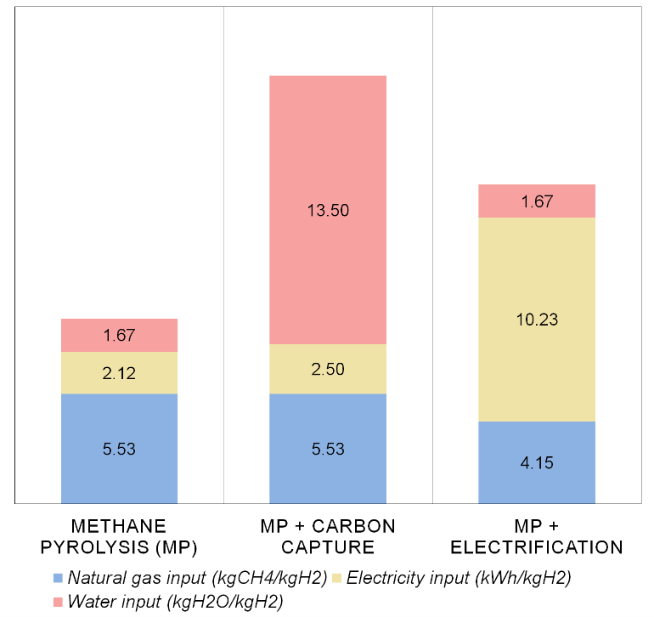
Results of the levelized cost and carbon intensity of hydrogen per case appear in Figure 1, while Figure 2 evaluates the major process inputs like natural gas, electricity and water.
The cost for the three processes is similar to SMR with an emissions intensity more than 50% lower with gas heating. By applying carbon capture or electric heating, emissions intensity is in the range of 0.1-0.4 kgCO2/kgH2. The additional equipment and more costly heat source increase the levelized costs. In the case of gas heating with carbon capture, emissions intensity is reduced by an order of magnitude relative to the gas heating only process, but results in a 13% increase in levelized cost.
Opportunities and barriers: Economic and technical challenges of methane pyrolysis
The implementation of methane pyrolysis faces several technical and economic challenges that limit its large-scale adoption. One of the main technical constraints is the complexity of its reactor design, which affects scalability and operational efficiency. Currently, most methane pyrolysis projects are in the technology readiness level (TRL) range of 4 to 9, with existing initiatives largely limited to small-scale facilities (B.C. Centre for Innovation and Clean Energy, 2024). Other key technical barriers include the heat supply, the formation and accumulation of solid carbon (carbon clogging), and the reactor lifespan (Chan et al., 2024; Sánchez-Bastardo et al., 2021). Among the most critical challenges is carbon clogging, which can hinder reactor performance and longevity; proven reactor designs are essential to mitigate this issue and improve the technical feasibility of methane pyrolysis at an industrial scale.
In addition to technical challenges, the financial viability of methane pyrolysis depends on the potential value of the carbon by-product. Several carbon-based products are generated with different operating temperatures and reactor designs, such as carbon black, graphite and nanoparticles (Lee et al., 2025; Prabowo et al., 2024; Sánchez-Bastardo et al., 2021). Capturing this added value from carbon products can help offset hydrogen production costs and improve the overall competitiveness of methane pyrolysis.
Next steps: Exploring hydrogen market for future energy systems
Future work should focus on comparing methane pyrolysis with other hydrogen production technologies, such as electrolysis, steam methane reforming with carbon capture, and biomass gasification, within the context of British Columbia’s energy landscape. This comparative assessment will help determine the most suitable regional pathways based on emissions, energy inputs, resource availability, and energy infrastructure. Additionally, estimating future hydrogen demand across key sectors, including transportation, industry, and power generation is essential to align production strategies with decarbonization goals. A detailed demand forecast will support infrastructure planning and guide investment priorities for a low-carbon hydrogen economy in British Columbia.
References
B.C. Centre for Innovation and Clean Energy. (2024). The Potential for Methane Pyrolysis in B.C. Report.
Brandon, N. P., & Kurban, Z. (2017). Clean energy and the hydrogen economy. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 375(2098), 20160400. https://doi.org/10.1098/rsta.2016.0400
Chan, Y. H., Chan, Z. P., Lock, S. S. M., Yiin, C. L., Foong, S. Y., Wong, M. K., Ishak, M. A., Quek, V. C., Ge, S., & Lam, S. S. (2024). Thermal pyrolysis conversion of methane to hydrogen (H2): A review on process parameters, reaction kinetics and techno-economic analysis. Chinese Chemical Letters, 35(8), 109329. https://doi.org/10.1016/j.cclet.2023.109329
Incer-Valverde, J., Korayem, A., Tsatsaronis, G., & Morosuk, T. (2023). “Colors” of hydrogen: Definitions and carbon intensity. Energy Conversion and Management, 291, 117294. https://doi.org/10.1016/j.enconman.2023.117294
Korányi, T. I., Németh, M., Beck, A., & Horváth, A. (2022). Recent Advances in Methane Pyrolysis: Turquoise Hydrogen with Solid Carbon Production. Energies, 15(17), 6342. https://doi.org/10.3390/en15176342
Lee, Y. H., Kang, H., Kim, S., Yang, G., Yang, S., Oh, J.-H., & Choi, S. (2025). Structural transformation of solid carbons produced from the methane pyrolysis process for turquoise hydrogen production. International Journal of Hydrogen Energy, S0360319924055903. https://doi.org/10.1016/j.ijhydene.2024.12.377
Marquardt, T., Bode, A., & Kabelac, S. (2020). Hydrogen production by methane decomposition: Analysis of thermodynamic carbon properties and process evaluation. Energy Conversion and Management, 221, 113125. https://doi.org/10.1016/j.enconman.2020.113125
Parkinson, B., Tabatabaei, M., Upham, D. C., Ballinger, B., Greig, C., Smart, S., & McFarland, E. (2018). Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals. International Journal of Hydrogen Energy, 43(5), 2540–2555. https://doi.org/10.1016/j.ijhydene.2017.12.081
Prabowo, J., Lai, L., Chivers, B., Burke, D., Dinh, A. H., Ye, L., Wang, Y., Wang, Y., Wei, L., & Chen, Y. (2024). Solid carbon co-products from hydrogen production by methane pyrolysis: Current understandings and recent progress. Carbon, 216, 118507. https://doi.org/10.1016/j.carbon.2023.118507
Sánchez-Bastardo, N., Schlögl, R., & Ruland, H. (2021). Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Industrial & Engineering Chemistry Research, 60(32), 11855–11881. https://doi.org/10.1021/acs.iecr.1c01679
Timmerberg, S., Kaltschmitt, M., & Finkbeiner, M. (2020). Hydrogen and hydrogen-derived fuels through methane decomposition of natural gas – GHG emissions and costs. Energy Conversion and Management: X, 7, 100043. https://doi.org/10.1016/j.ecmx.2020.100043
Cost and emissions intensity of hydrogen from thermal pyrolysis of natural gas in BC PDF
