UVic Science: A Health-y Faculty

April 7, 2024 | by Nicole Crozier
World Health Day, celebrated every year on April 7, is a global health awareness day that aims to raise awareness around various health issues. Here in the Faculty of Science, we’re working some of those issues every day. Our researchers are developing new therapeutics and treatments for diseases, uncovering the molecular mechanisms by which viruses and bacteria are operating, evaluating the effectiveness of public health interventions, uncovering the physiology of diseases and much more.
This World Health Day, explore some of the health-related research happening in our Faculty, across five of our six departments.
Understanding the role of insulin in female reproductive health

Polycystic ovary syndrome (PCOS) is a common endocrine disorder, estimated to affect ~10% of women worldwide. While PCOS is named after the cysts that form on the ovaries of some people with the condition, it’s characterized by a wide range of symptoms that can also include disrupted hormone levels, irregular menstrual cycles, infertility and metabolic changes such as elevated insulin. Despite the prevalence of the disorder, the exact causes are still unclear and there is currently no cure.
A frequent feature of PCOS is abnormally elevated insulin levels, and this is where Nicole Templeman, assistant professor of biology, is focusing her research. She is currently working to better understand the role of insulin in female reproductive health and to determine whether preventing an elevation in insulin levels can effectively protect against the progression and severity of PCOS.
“PCOS is a multifactorial disorder with symptoms that differ widely between individuals. While this makes it difficult to pinpoint its causes, it also means this disorder is well-suited to individualized treatments that are based on personal history and symptoms. To advance that goal, we are examining the effects of specifically targeting one symptom of PCOS, elevated insulin, to see the full extent to which it exacerbates the disorder.”
- Nicole Templeman, Tier 2 Canada Research Chair
Using a mouse model with genetically reduced insulin and induced PCOS features, Templeman and her research group is testing how suppressing insulin levels affects a spectrum of reproductive and metabolic characteristics of PCOS. They’re looking to see if limiting insulin will alleviate the severity of PCOS-like pathophysiology, improve fertility, mitigate pregnancy complications and reduce the incidence of gestational diabetes.
“We already know that it’s possible to lower insulin levels through lifestyle changes. My hope is that our research could inform strategies to better manage or even prevent some of the health impacts of PCOS by limiting excess insulin.”
Templeman is a Tier 2 Canada Research Chair in Cell Biology and a Michael Smith Health Research BC Scholar. Her PCOS research is supported by the Women’s Health Research Institute.
Evaluating the impact of contact tracing during a disease outbreak
In the last 25 years, there have been several major disease outbreaks that have had a severe impact on population health, including SARS, H1N1 and most recently, COVID-19. In each case, the government has implemented a series of public health interventions, such as social distancing, masking, prohibiting large gatherings and contact tracing, to try and limit the spread of the disease.
Understanding the effectiveness of these interventions is important and can help us understand where to direct our time and resources during an outbreak. UVic statistician Laura Cowen and mathematician Junling Ma are developing mathematical models to help evaluate the effectiveness of one of those interventions: contact tracing.
“Contact tracing, which involves identifying and isolating individuals at high risk of infection by tracking the contacts of diagnosed patients, can be an effective strategy for controlling epidemics. It typically helps to reduce disease transmission among the traced contacts, reducing further spread of the disease. However, it can be a time-consuming process, especially as the number of cases increases.”
- Laura Cowen, mathematics and statistics professor
Mathematical models are important tools for studying the dynamics of infectious diseases and evaluating control measures. In this case, Cowen and Ma are using mathematical models to evaluate the effectiveness of contact tracing in the early stage of an epidemic. Their model disentangles the effects of contact tracing from the effects of other public health measures. Their model also accounts for asymptomatic transmission and adopts a network model approach, rather than assuming that every individual has the same probability of contacting every other individual.
“A large part of the development of our model was determining what data we needed in order to accurately assess the effect of contract tracing,” says Cowen. “Ultimately, we determined that if we know new case counts, the number of cases identified by either contact tracing or voluntary tests and counts of symptomatic onset, we can evaluate contact tracing.”
This research has been funded by the Michael Smith Foundation for Health Research, the Victoria Hospitals Foundation and the National Science and Engineering Research Council (NSERC).
Using plants for drug discovery

PhD student Sarah Lane is a plant biologist at heart, but she hasn’t let that stop her from working on human health related projects. She is currently researching the potential of novel therapeutics sustainably sourced from plants, with a focus on iron overload conditions.
There are lots of different human diseases that are worsened by iron overload, such as sickle cell anemia, beta-thalassemia and Parkinson’s disease. In these diseases, iron may accumulate where it isn’t needed, be overreactive and cause tissue damage. In Parkinson’s disease, for example, an accumulation of iron occurs in the region of the brain responsible for fine motor movement and has been linked to symptoms like tremors as the disease progresses.
While humans sometimes struggle with managing iron, plants generally don’t.
“Plants have the iron situation on lockdown. They make lots of different molecules that interact with iron, and they can absorb iron without it being super reactive. If we can get those molecules into people, they may help.”
- Sarah Lane, PhD student in the Centre for Forest Biology
Lane’s research focuses on identifying plant metabolites that interact with iron and evaluating how they react and what they do in human cells. While most plant-based medicinal research involves grinding up plants and extracting molecules to see if they have an effect on disease, Lane takes it one step back and creates conditions that induce the plant to create the metabolites she is interested in. After identifying and isolating the target metabolites, she tests them in cell culture to determine if they have the type of activity she is looking for in that environment.
“Ultimately, this is a drug discovery process, but without the chemistry lab,” says Lane. “Drug discovery is typically super expensive and takes a lot of work. There are lots of things that plants do already that would take us years to figure out in a pharmaceutical lab. Using plants can help make drug discovery faster and more sustainable.”
Lane’s research, conducted under the supervision of Jürgen Ehlting, has been supported by funding from the Thalassemia Foundation of Canada, a UVic Health Grant and the National Science and Engineering Research Council (NSERC).
Enhancing genomic medicine with new polymer nanoparticles
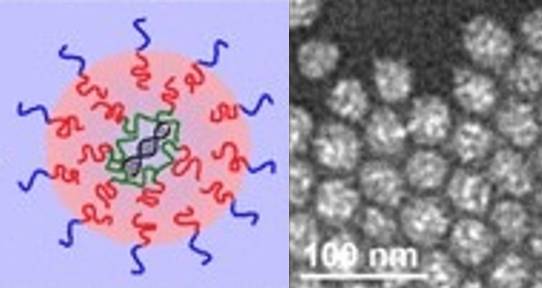
Nucleic acids, such as DNA and RNA, are key polymers in the human body, and are involved in the storage and expression of genomic information. DNA provides the code for a cell’s activities while RNA converts that code into the proteins that carry out cellular functions.
Modern medicine is finding many uses for nucleic acids in the treatment of diseases, including in gene therapy and vaccines. However, the nucleic acids need to be delivered safely and effectively to the correct locations in the body. Developing technology that can do so is a research focus for UVic chemistry professor Matthew Moffitt.
“I believe that polymer nanoparticles (PNPs) are the best way to deliver nucleic acids and that’s the technology we’re interested in developing in my lab. Viral particles have been used in both in the lab and in clinical trials, but there are often side-effects, and the limited quantity of nucleic acid they can carry makes many applications impossible. Lipid nanoparticles have also been used successfully, including in recent RNA-based COVID-19 vaccines, but they don’t have the stability, ease of functionalization and variability of properties that PNPs do.”- Matthew Moffitt, chemistry professor
Moffitt is currently developing a new type of gene delivery PNP, called a “polyplex-in-hydrophobic core” (PIHC) micelle. In this type of PNP, the nucleic acid cargo is surrounded by a hydrophobic polymer core, which itself is surrounded by a hydrophilic polymer shell. The PIHC micelles are potentially safer and more effective vectors for vaccines and other gene-based medicines as the structure of the PIHC micelle provides enhanced protection for the nucleic acids against attack and degradation by enzymes in the blood stream and minimizes the exposure of healthy cells to the potentially harmful content of the PNP.
Moffitt’s long-term goal is to get this material into the hands of as many licensing partners as possible. He is currently working with several different local biotechnology companies to refine the PIHC micelle, obtain pre-clinical data and determine what will be required for future clinical research and regulatory approvals.
“This technology has a high potential to have a major impact on gene therapy and vaccine delivery,” says Moffitt. “We’re working to get multiple industry users involved in the testing and development as early as possible, so that we can get the technology into the hands of potential licensing partners and into regular use as soon as we can.”
Moffitt’s research is supported by an Idea to Innovation grant from the National Science and Engineering Research Council (NSERC).
Examining the co-infection of Influenza A and Staphylococcus aureus

The flu is a commonly known seasonal illness that most people aren’t too worried about. Every year, there are almost a billion infections worldwide, but most cases are uncomplicated, resolve themselves without medical intervention and only last 1-7 days.
However, ‘most’ is not ‘all.’ Those with the flu, caused by the Influenza A Virus, are susceptible to co-infection. In these cases, the virus and a bacteria, most commonly Streptococcus pneumoniae or Staphylococcus aureus, are both present and synergistically enhance each other, to the detriment of the host. UVic biochemistry and microbiology professor Mariya Goncheva is working to better understand this synergy.
“When we look at hospitalized patients with the Influenza A Virus, bacterial co-infection is present in 20-40% of cases. Mortality rates during co-infection can be as high as 35%. The flu isn’t innocuous and it can open the door for other pathogens.”
- Mariya Goncheva, biochemistry and microbiology professor and Tier 2 Canada Research Chair in Virology
Goncheva’s research focuses on the bacteria S. aureus, which, prior to COVID-19, caused more deaths than any other infectious agent in the United States and is currently number four on the WHO’s list of bacteria that require urgent development of new antibiotics. The negative effects of S. aureus are increased during co-infection and can include abnormal immune system responses, the destruction of the blood-air barrier in the lungs, and direct virus-bacteria interactions.
Goncheva focuses on these virus-bacteria interactions and is specifically working to identify the molecular mechanisms through which the bacteria affects the replication of the virus and the progress of the illness. For example, she is examining how the presence of the bacteria changes the composition of the virus, the scope of bacterial proteins that can impact the virus and if long-term genetic mutations are selected for in the virus during co-infection.
“By learning more about what happens on the molecular level during co-infection, we’re hoping we can help inform best practices for co-infection management in the clinic,” says Goncheva. “The goal is for our research to provide new targets for the development of therapeutics and result in better patient outcomes—ultimately saving lives.”
Goncheva’s research is supported by the BC Lung Foundation and the National Science and Engineering Research Council (NSERC).
Increasing the effectiveness of cancer radiotherapy

Radiation therapy is currently one of the main treatment modalities for cancer. UVic medical physicist Devika Chithrani is working to increase the effectiveness of the cancer radiotherapy, while simultaneously reducing the side effects.
“We are currently at the limit of the dosage of radiation we can give to patients,” says Chithrani. “While radiation therapy does save lives by damaging cancer cells, it also damages healthy cells in the surrounding treatment area, so there’s a careful balancing act involved when it comes to treatment.”
Chithrani’s research aims to tip the scales in favour of radiation. She’s focused on enhancing the amount of radiation given directly to the tumor and maximizing its effect, but also minimizing the dose received by healthy tissue. Currently, she is exploring the potential of a new radiotherapy that would include adding a unique combination of two radiosensitizing agents known to enhance the effect of radiation: Gold nanoparticles and docetaxel.
“Gold nanoparticles target tumour cells and react with the radiation to kill those cells only,” says Chithrani. “Docetaxel, a chemotherapeutic that can be delivered directly to the tumour via a lipid nanoparticle system, makes the tumour cells more sensitive to the radiation. Combining these two treatments with radiotherapy has the benefit of better targeting and increased effectiveness.”
- Devika Chithrani, medical physicist
Chithrani is not just testing new treatments, but is also implementing new ways to test these therapeutics, using 3-dimensional tissue models in the lab. These models allow her to examine the effect of the treatment before testing in animal models, making the process of testing new therapies both faster and more cost efficient.
Chithrani’s research is supported by the Canadian Institute for Health Research (CIHR), Networks of Centers of Excellence and the National Science and Engineering Research Council (NSERC).
