Offering a new hypothesis for the cause of Alzheimer’s disease
Alzheimer’s is a complex neurodegenerative disease affecting millions of people worldwide. Yet despite extensive research, our understanding of why the disease leads to cognitive decline and memory loss remains incomplete. Now, researchers in the University of Victoria’s Division of Medical Sciences have published an article in Nature Communications that could critically improve our understanding of what causes Alzheimer’s disease.
 Many well-accepted explanations of the disease include oxidative stress, inflammation, and the buildup of protein clumps (e.g., of amyloid-beta and tau). However, in the new paper, first-author and MSc student Victor Lau (Tremblay Lab; pictured), Dr. Leanne Ramer (Simon Fraser University), and senior author Dr. Marie-Ève Tremblay propose a new hypothesis: the progression of Alzheimer’s disease must involve maladaptive, senescent cell buildup.
Many well-accepted explanations of the disease include oxidative stress, inflammation, and the buildup of protein clumps (e.g., of amyloid-beta and tau). However, in the new paper, first-author and MSc student Victor Lau (Tremblay Lab; pictured), Dr. Leanne Ramer (Simon Fraser University), and senior author Dr. Marie-Ève Tremblay propose a new hypothesis: the progression of Alzheimer’s disease must involve maladaptive, senescent cell buildup.
Cellular senescence is a phenomenon in which cells stop dividing but do not die off when they should. Along with being resistant to cell death, senescent cells can also survive environment stressors like those created by amyloid-beta, tau, and oxidative stress. Instead, they internalize and semi-degrade these stressors. The senescent cells then spread the accumulated, partially digested protein aggregates to nearby cells.
The authors suggest that this distribution of amyloid-beta, inflammation, and especially, tau by senescent cells provides and sustains a disease environment that spreads locally and evolves into the progression of Alzheimer’s disease. They also propose that microglia, the resident immune cells of the brain, are the last and most critical defense against cognitive decline seen in Alzheimer’s. When microglial senescence accumulates, they predict the disease will finally progress to later-affected brain regions via spreading and buildup of tau aggregates.
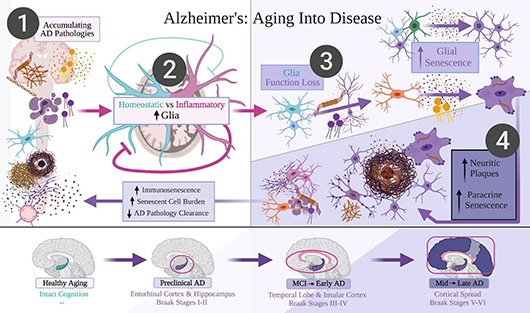 Victor and his co-authors suggest that this new understanding of the disease could open up new avenues for treatment and prevention, as well as help with determining the difference between subjective cognitive aging and dementia. Their review paper provides a comprehensive overview of the evidence supporting the new hypothesis, though the researchers recognize that further study is needed to fully confirm or reject their theory and to fully understand the complex mechanisms underlying Alzheimer's disease. Of course, they hope that testing the new empirical predictions and senolytic medications, which kill senescent cells, suggested by their hypothesis will allow for breakthroughs on treating Alzheimer’s disease, dementia, and other neurodegenerative diseases.
Victor and his co-authors suggest that this new understanding of the disease could open up new avenues for treatment and prevention, as well as help with determining the difference between subjective cognitive aging and dementia. Their review paper provides a comprehensive overview of the evidence supporting the new hypothesis, though the researchers recognize that further study is needed to fully confirm or reject their theory and to fully understand the complex mechanisms underlying Alzheimer's disease. Of course, they hope that testing the new empirical predictions and senolytic medications, which kill senescent cells, suggested by their hypothesis will allow for breakthroughs on treating Alzheimer’s disease, dementia, and other neurodegenerative diseases.
The review paper also forms the basis of Victor’s current research projects, which aim to assess if dark microglia—a novel microglial state discovered in the Tremblay lab and observed in many diseases—correspond to dystrophic microglia. Dystrophic microglia are proposed to be senescent and a major critical driving force in Alzheimer’s progression. The outcome of Victor’s work, along with research from Dr. Tremblay and other Tremblay Lab members, may provide further insight about dystrophic and dark microglia in multiple neurodegenerative diseases.
“I feel very lucky to be supported and be a part of something bigger than myself, of something that can hopefully directly impact people with lived experience,” Victor says. “While the foundation of my motivations will always first derive from dementia, it’s been exciting to be on the edge of a new field and research topic—one that branches out into more neurodegenerative diseases likely involving senescence.”
